Introduction
Diagnosis and treatment of snoring and obstructive sleep apnea (OSA) is an interdisciplinary matter. In addition to Otorhinolaryngology it includes Internal Medicine, Pneumology, Cardiology, Dentistry, but also Pediatrics and Neurology. The mandibular advancement devices (MAD) are now considered a reliable and non-invasive therapy option in the treatment of habitual snoring and obstructive sleep apnea (OSA) (Lit. 1, 2).
The American Academy of Sleep Medicine (AASM) has recognized and recommended MAD systems since 2006. The position paper published in 2015 identifies MAD as a first line therapy for the patients with snoring and mild to moderate OSA (Apnea Hypopnea Index [AHI] < 30/h) and a Body Mass Index (BMI) of < 30 kg/m2) in addition to Continuous Positive Pressure Breathing (CPAP) at night (Lit. 3, 4). The American Academy of Otolaryngology -Head and Neck Surgery, originally published a position statement in 2014, and updated it in 2019, strongly supporting the Otolaryngologist as uniquely qualified to serve the needs of patients with airway issues contributing to OSA with treatments and therapy solutions including MADs (Lit. 5)
It is known that CPAP therapy is not accepted by every patient. The CPAP-Compliance is less than 50% compared to up to 80% with MAD (Lit. 5). In the case of severe OSA (AHI > 30/h), CPAP treatment can be combined with MAD to reduce the respiratory pressure and thus increase acceptance (Lit. 3).
How do mandibular advancement devices work? MAD displace the lower jaw forward, making it more difficult to open the mouth and the tongue to fall back, thus increasing the volume of the upper airways. In addition, the hyoid is shifted upwards and forward and the velum is pulled forward by tightening the musculus palatoglossus. All this prevents collapse during sleep and reduces the obstruction.

The first studies on MAD in sleep apnea were conducted by Prof. Dr. Karlheinz Meier-Ewert, a German neurologist/psychiatrist and sleep physician, who first presented this form of therapy in 1984 (Lit. 5). For the nightly advancement of the lower jaw he used Monoblock splints, which he called Esmarch's devices. The MADs have undergone a positive development in recent years, namely from the one-piece chunky Esmarch splint to elegant small two-piece models. With the two-piece MADs, the top and bottom trays are connected with different fasteners, such as frontal screws or hooks, side connectors or telescopes. This allows them to slide against each other and unlike the monobloc devices after adjustment the lower jaw can be moved forward until the desired effect is achieved.
Prefabricated thermoplastic MAD
While so-called "Boil & Bite” MADs have been available for many years, advancements in materials and design have yielded thermoplastic appliances that are of very high quality, clinically sophisticated and yet are far more affordable than the historically lab-made alternative (Figures 1 - 3). The advantage of this class of appliance is that it is very efficiently custom-fit and delivered in a single, short office visit, by heating the prefabricated appliance sections in a hot water bath and then easily formed to the individual teeth and jaw formation. Once cooled, the trays retain the precise impression and individual specific bite registration. The patient can initiate therapy the same night. The fitting process can be done in an ENT office by the physician or also by a trained nurse, MA or PA.
These thermoplastic MADs are available as Monoblock or two-piece devices, like the SomnoGuard AP2 (Fig 1). The new SomnoGuard SPX , for example, is much smaller than the conventional thermoplastic splints (Fig. 4). It offers a higher using comfort due to considerably less thermoplastic material and highly refined design. The new material has a so-called shape memory effect, which allows the device to be adjusted repeatedly, as it returns to its original shape in hot water and can be reshaped. It fits to all sizes of jaws and encloses the front teeth only slightly, so that little pressure is exerted on the teeth, which again considerably increases the using comfort. The ability to reshape the material at any time during the useful life of the appliance also presents a substantial benefit over lab-made appliances as they can accommodate a change in the patient’s dental circumstances by remolding the impacted tray without the need to produce a new custom appliance tray.
A further advantage is that the user can adjust the necessary mandibular advancement with millimeter precision evenly through the use of lateral connecting pieces (turnbuckle connecters or straps in different sizes) or the precise titration screw mechanism available in other models.
Being FDA cleared for both Snoring and Obstructive Sleep Apnea, the SomnoGuard can play many roles in the ENT practice, serving as a first line alternative as compared to CPAP for patients identified to suffer with Mild to Moderate OSA with a sleep study. It is also an effective, efficient and far more affordable therapy solution for snoring than lab made appliances which few will invest in for the treatment of snoring alone, or those resistant to participating in a diagnosis of suspect OSA. The thermoplastic MADs are also suitable as trial devices. Before investing in a more expensive custom MAD, it is recommended that you first select this less expensive option for testing. This will show whether the patient tolerates a MAD at all and whether the device is an effective therapy option.
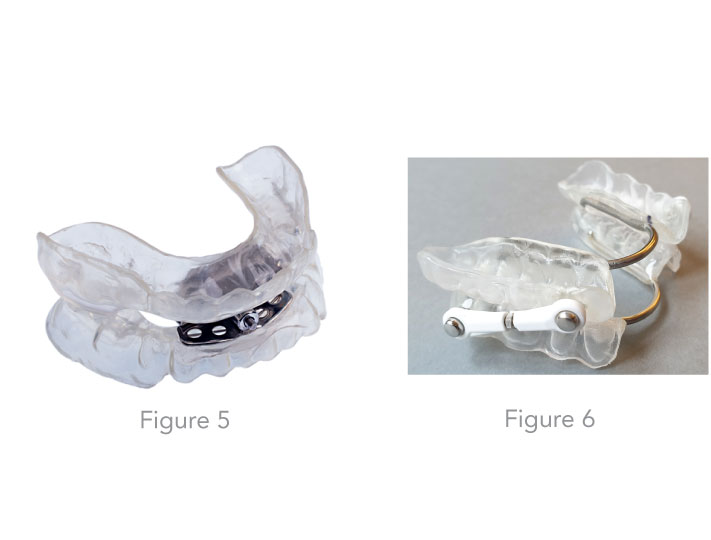
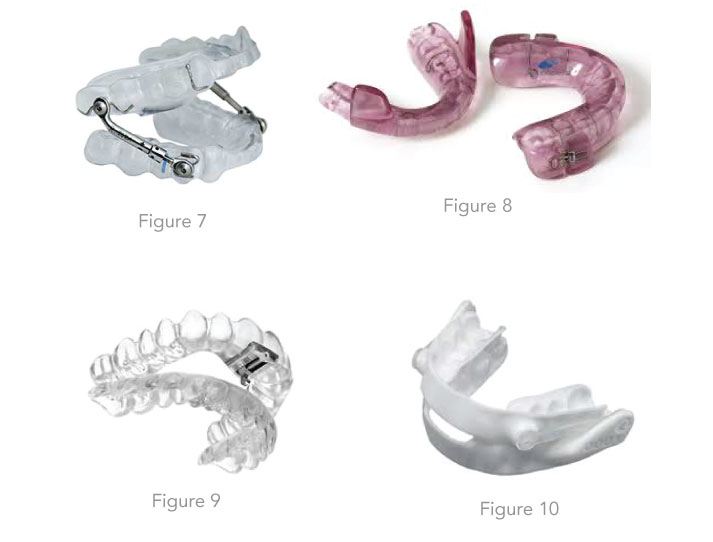
Insight on the custom fabrication process of MADs in the dental laboratory Depending on the findings, the dentist selects the most suitable device type for the patient (Figures 5 - 8). Individual impressions of the upper and lower jaws are required for the fabrication of the MAD. From these impressions, exact hard models are made so that the devices later be fit on the dental arch. In the past few years some dentists have begun utilizing a new scan technology for the impressions which is more precise than a manual one. Then a special bite registration follows, in order to specify precisely to the dental technician in which mandibular advancement position the MAD should be made. Various factors such as the bite position (open bite, deep bite) and the inclination of the masticatory plane must be taken into account. Custom-made devices can be worn for up to 3 to 5 years, depending on the patient's care, oral hygiene and the presence of teeth grinding. Double layer materials are used for the newer ones. A softer and flexible inner lining provides a comfortable wearing comfort and the outer hard plastic layer provides more stability.
Lately there are some newer nylon custom made devices in the market which are made with 3-D Printing technology after intraoral CAD/CAM Scan (Fig 10).
Examination before a fitting
A thorough examination of the teeth, jaw and temporomandibular joints should be performed before a MAD fitting. This must be done to determine whether there are enough healthy teeth to anchor the devices. Existing periodontitis and dental issues must be detected and treated beforehand. The mobility of the temporomandibular joints at maximum advancement is measured and checked for possible pain. The findings of the buccal mucosa, tongue size in relation to the oral cavity, pharyngeal findings (soft palate, uvula, Mallampati degree) and the size of the tonsils should also be documented. A progeny or retrogene is to be recorded in the form of a photo documentation.
At the beginning of the treatment, the degree of mandibular advancement, if any should be carefully assessed and advancement managed precisely as needed, with each patient being unique. With increasing habituation, advancement can be increased step by step up to two thirds of the maximum possible advancement, if necessary, to increase the effect. During the acclimatization period, which can last up to two weeks, hypersalivation, xerostomia, temporomandibular joint problems and toothache are possible. In very rare cases, permanent tooth misalignment may occur (Lit. 7).
Controls after the fitting
For OSA patients, outpatient monitoring (Polygraph) should be performed within three months of the start of MAD therapy in order to check the treatment outcome and whether the therapy goal has been achieved. If necessary, the setting of the MAD is changed and the lower jaw is moved further forward.
Conclusion
MAD is an evidence-based and internationally guideline compliant therapy for the interdisciplinary treatment of snoring, mild to moderate obstructive sleep apnea and patients with CPAP intolerance. It can be used either as first-choice therapy, as a treatment alternative in case of CPAP intolerance or together with CPAP as a combination therapy. For an effective MAD therapy in obstructive sleep apnea, it is recommended that less-costly, efficient, effective, convenient, readily available and far more affordable prefabricated thermoplastic MAD are first used to deliver the health benefits and determining whether the patient tolerates a MAD and if it presents an effective therapy solution.
Literature
-
Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Effects of an oral appliance with different mandibular protrusion positions at a constant vertical dimension on obstructive sleep apnea. Clin Oral Investig 2010;14:339-345.
-
Almeida FR, Lowe AA. Principles of oral appliance therapy for the management of snoring and sleep disordered breathing. Oral Maxillofac Surg Clin North Am 2009;21: 413-420.
-
Randerath WJ et al. Konsensuspapier zur Diagnostik und Therapie schlafbezogener Atmungsstörungen bei Erwachsenen. Pneumologie 2014;68:106-123.
-
Kushida CA, Morgenthaler TI, Littner MR et al (2006) Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep 29(2):240–243 5.Pantin CC1, Hillman DR, Tennant M., Dental side effects of an oral device to treat snoring and obstructive sleep apnea Sleep. 1999 Mar 15;22(2):237-40.
-
Meier-Ewert K, Schäfer H, Kloß W (1984) Treatment of sleep apnea by a mandibular protracting device. 7th Europ Sleep Res Soc Congress, Munich (Abstract)
-
Menn SJ, Loube DI, et al. The mandibula repositional device: role in the treatment of obstructive sleep apnea. Sleep 1996; 19: 794-800
Article by: Dr. med. Fahri Yildiz Otorhinolaryngologist and Sleep Medicine, Cologne/Germany